What magical uses do rare earth elements have? (Ⅰ) ——Overview of rare earth element applications
Rare earths are indispensable materials for new technologies and new materials. They are commonly known as ‘industrial gold’ and are important strategic resources. This article aims to help readers understand the wonderful uses of rare earth elements.
I. How many kinds of metal elements are included in rare earth?
What are rare earths? ‘Rare’ means rare; ‘earth’ is usually used to refer to solid oxides that are insoluble in water, such as aluminum oxide, called clay, and magnesium oxide, called pyrophyllite; rare earth refers to certain metal oxides. To be precise, rare earth is a general term for 17 metal elements in the periodic table, including ‘scandium’, ‘yttrium’ and ‘lanthanide elements’. The oxides of these 17 rare earth elements are always mixed together.
Rare earth elements: scandium, yttrium and lanthanide series a total of 17 elements
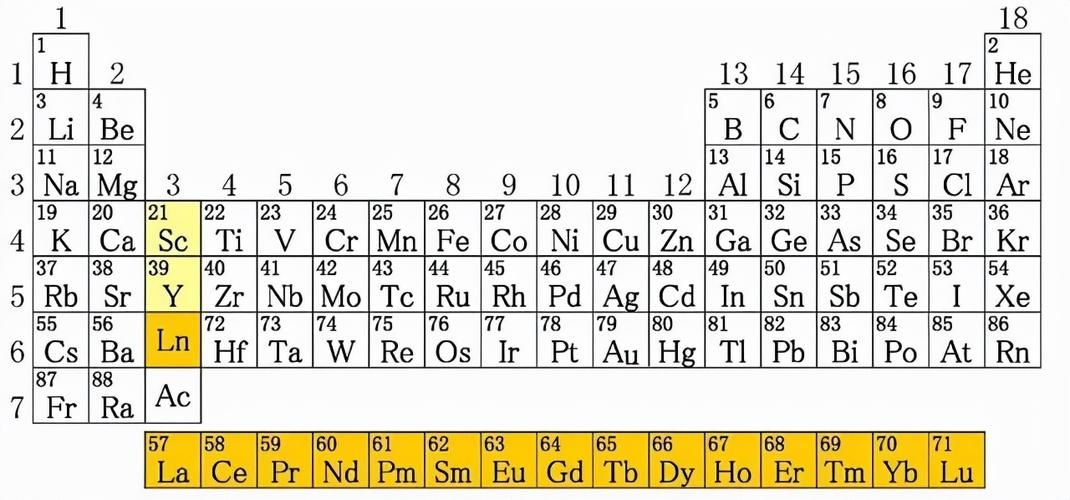
Rare earth elements are located in the third column of the periodic table, namely scandium (SC), yttrium (Y), and lanthanum (La).
However, Lanthanum stands for ‘Lanthanide’, which is a group of 15 elements: Lanthanum (La), cerium (Ce), praseodymium (Pr), neodymium (Nd), promethium (Pm),
Samarium (Sm), Europium (Eu), Gadolinium (Gd), Terbium (Tb), Dysprosium (Dy),
Holmium (Ho), erbium (Er), thulium (Tm), ytterbium (Yb), lutetium (Lu).
Therefore, there are 17 rare earth elements in total, of which those with active properties and light atomic mass are called ‘light rare earth elements’, a total of 8:
Lanthanum (La), cerium (Ce), praseodymium (Pr), neodymium (Nd),
promethium (Pm), Samarium (Sm), Europium (Eu), Gadolinium (Gd).
There are seven elements with heavier atomic masses, known as ‘heavy rare earth elements’:
Terbium (Tb), Dysprosium (Dy),Holmium (Ho), erbium (Er),
thulium (Tm), ytterbium (Yb), lutetium (Lu).
Introducing some ‘rare earth properties’
Rare earth elements have many special properties, a few of which are introduced below.
1. Generally speaking, rare earth elements are more active than ‘aluminum’.
They react with oxygen to form oxides, react with hydrogen to form hydrides, form compounds with many non-metallic elements and react with many acids and bases to form ‘rare earth salts’;
2.As the ‘atomic number’ decreases, the‘activity’ increases; as the ‘atomic number’ increases, the ‘metallicity’ decreases.
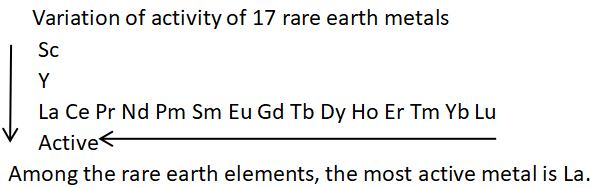
3. Alloys of ‘lanthanide elements’ and ‘transition metals’ are important hydrogen storage materials, such as LaNi5.
It has a strong hydrogen storage capacity under several atmospheres of pressure and can release hydrogen by reducing the pressure.
4. Rare earth metals and their oxides, hydroxides, and carbonates are originally insoluble in water. Still, they can react with hydrochloric acid to produce ‘rare earth chlorides’, which are easily soluble in water, and the higher the temperature, the greater the solubility.
5.Rare earth metals, their oxides and hydroxides react with sulfuric acid to form ‘rare earth sulfates’, and their solubility in water also has a strong regularity.
6.‘Rare earth sulfates’ can form ‘complex salts’ with ‘alkaline earth metal sulfates’. The solubility of these complex salts varies greatly and they are easy to separate.
The above solubility characteristics will be used in the separation and extraction technology of rare earth elements.
7.Rare earth elements are relatively active, which can easily fuse with other crystals, making the crystal structure small and dense.
8.The atomic structure of rare earth elements is quite special and has multiple ‘energy levels’. How do we know this phenomenon? When rare earth elements are illuminated with a certain light to observe their spectra, it can be found that there is more than one spectrum, which shows that the atoms of rare earth elements have multiple "energy levels".
9. There are about 30,000 absorption spectra of all rare earth element atoms or ions, all of which are ‘narrow-band spectra’ from infrared to ultraviolet. Since the frequency of the absorption spectrum is also the frequency of the excitation-emission spectrum, rare earth elements can emit special light when irradiated with excitation light. For this reason, rare earths have become a huge treasure trove of luminescent materials, from which many new luminescent materials can be discovered. Moreover, rare earth elements have low luminescence conditions, strong luminescence ability, high luminescence efficiency, and pure light color.
What are the uses of rare earths?
Rare earth elements are known as ‘industrial gold’ and ‘industrial vitamins’, which means that they are important and practical and are everywhere in the industry. When they are used in a material, they will magically improve the performance and production efficiency, increase scientific and technological content, and promote technological progress of that material. More specifically, modern high-tech products cannot do without the participation of rare earths. The following is a summary of the application of rare earths in several fields.
1.Usage in metallurgical industry
‘Rare earth ferrosilicon alloy’ and ‘rare earth silicon-magnesium alloy’ are used as spheroidizing agents in cast iron, which can transform the strip-shaped ‘iron-carbon crystals’ into ‘spheres’, thus becoming ‘ductile iron’, which greatly improves the processing performance and mechanical properties of cast iron. It plays a huge role in the machinery manufacturing industry.
Adding rare earth metals to non-ferrous alloys, such as magnesium, aluminum, copper, zinc, and nickel, can improve the physical and chemical properties of the alloys at room and high temperatures.
2.Application in strong magnetic materials
The well-known ‘king of permanent magnets’ is the ‘NdFeB’ alloy, in which rubidium is a rare earth element. Strong permanent magnets have a wide range of uses, like in ‘permanent magnet motors’, ‘magnetic resonance imaging’, and ‘electrical components’.
3.Usage in petrochemical industry
In the petrochemical industry, molecular ‘cracking’ and ‘polymerization’ are very common, but they all require the use of catalysts. Adding rare earth elements to the catalyst can double the efficiency; making the catalyst into a "molecular sieve catalyst" with "good permeability" and "large contact area" can double the catalytic effect.
In chemical printing and dyeing, the use of rare earths can make dyeing stable and bright.
4.Usage in glass ceramics
‘Special optical glass’ can be made by adding rare earth elements, some of which can pass infrared rays, absorb ultraviolet rays, resist heat, acid, and alkali, or block X-rays. These ‘high-grade optical glasses’ are widely used in civilian high-tech.
High-power ‘laser crystals’ must contain rare earth elements. This type of laser can be used in the mechanical processing, scalpels, etc.
Adding rare earths to ceramic glazes and porcelain glazes can not only make the sintered products present different colors and luster but also reduce the breakage of the glaze. Fine-grained rare earth oxides can be widely used as polishing agents for various types of glass.
5.Usage for optical fiber communication
By adding rare earths to optical fiber materials, signals can travel through the fiber with less loss and can travel farther.
By doping rare earths into optical fibers and irradiating them with LED lights, the doped rare earth elements can be stimulated to emit light. This principle can be used to make a ‘relay amplifier’ for optical fiber signal transmission, which we call "pump light". ‘Pump light’ can also be used to make high-power lasers.
6.Usage for electrochromic display
High-tech thin-film displays generally use "phosphors that display different colors", and these phosphors require the participation of different rare earth elements.
7.Used for radioactivity
Heavy rare earth elements can generally absorb neutrons and turn into corresponding isotopes, so they can participate in ‘uranium raw materials’ and control the fission rate. Those isotopes are also radioactive and have lower energy, so they can be used in ‘local chemotherapy’ and ‘radioactive display tracking’ in the medical field.
8.Usage in agriculture
Research results show that adding a small amount of rare earth to the soil can promote seed germination, increase the seed germination rate, and promote seedling growth; it can increase plant chlorophyll content, enhance photosynthesis, promote root development, and increase root nutrient absorption; it can improve the disease resistance, cold resistance, and drought resistance of crops; spraying fruits with an appropriate amount of rare earth mixture can improve the quality of the fruits. The reason is that the light emitted by rare earth elements under sunlight is beneficial to plant growth.
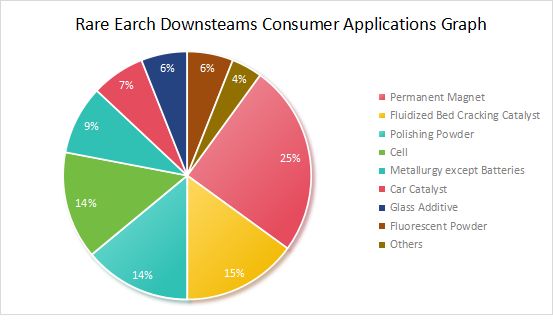
Rare earth application area consumption ratio

 EN
EN NL
NL FR
FR DE
DE JA
JA KO
KO PT
PT RU
RU ES
ES TR
TR